New drugs won’t be enough to fight multi-resistant bacteria. We also need to prescribe antibiotics more intelligently.
“I’ve seen patients die of an infection, especially in the service that treats severe burn victims, because no antibiotic would work on them,” says Yok-Ai Que, head of Education and Research at the Department of Fundamental Microbiology at the University of Lausanne. “Sometimes we have to put patients on an IV just to treat cystitis (inflammation of the bladder).”
What causes these tragedies? The growing number of antibiotic-resistant bacteria in Swiss hospitals.
E. coli, a common intestinal bacterium that can cause gastroenteritis and urinary infections, no longer reacts to fluoroquinolone, the antibiotic usually used to kill it off, in 20.5% of cases according to the Swiss Centre for Antibiotic resistance (Anresis). In 2004, the percentage stood at 10.3%. Another example is K. pneumoniae, a bacterium that causes respiratory infections, has become unresponsive to third-generation cephalosporins, a more recent antibiotic, in 11.2% of cases, up from 1.3% in 2004.
“Resistance to penicillin first emerged just nine months after the drug was discovered in 1947”, and resistance has only become worse with the overuse of antibiotics. At Swiss hospitals, the number of daily doses administered jumped 36% between 2004 and 2013. “They’re often mistakenly used for diseases caused by viruses instead of bacteria, like colds,” he says.
Outside Switzerland, the situation is no more reassuring. Turkey, Greece, France and the United States are the champions of antibiotics consumption, according to the European Centre for Disease Prevention and Control. “In countries where poor hygiene is wide- spread, like India, or common in hospitals, like in Italy and Greece, patients are often given antibiotics as a preventive measure”, says Patrice Nordmann, a professor from the Microbiology Unit at the University of Fribourg.
The use of antibiotics on chicken, pig and fish farms – to prevent the spread of infections when animals are kept in cramped living conditions – also builds resistance, as have globalisation and the growth in medical tourism. Patients are increasingly transferred between countries, and that spreads germs. “We think that the first ex- tended-spectrum beta-lactamases [enzymes that cause resistance to antibiotics] first came to French-speaking Switzerland when the victims of the 2002 Bali bombings were brought to the Lausanne University Hospital (CHUV),” says Didier Pittet.
“Travellers who go to countries like India often bring back resistant germs in their gut flora,” says Thierry Calandra, head of the Infectious Diseases Service at CHUV. These healthy carriers have no symptoms, but they can pass these bacteria on to other people with weakened immune systems. There are a few ways to fight these super-bugs. Simple measures, like improving hand hygiene and vaccinating high-risk patients, would prevent 30% of the 70,000 infections contracted every year at Swiss hospitals.
We also need to reduce the consumption
of antibiotics. “In Switzerland, antibiotics cannot be sold without a prescription,” says Karin Wäfler, an antibiotic resistance project leader at the Swiss Federal Office of Public Health (FOPH). “But there are no binding guidelines that define when antibiotics should be prescribed or which one is the most appropriate.”
Since 2004, Anresis has been collecting information on resistant bacteria and antibiotics prescribed at hospitals, but not in outpatient care.
To combat resistance, we have to better understand its prevalence and its distribution among the population.
Antibiotic-resistant bacteria in livestock such as pigs, chickens, and cows have been reported since 2006, but not the amounts of antibiotics administered. The Swiss Antibiotic Resistance Strategy (StaR), launched by the Federal Council in early 2016, “will fill those gaps by systematically recording all of that information,” says Karin Wäfler.
But that won’t be enough to stop the spread of these super-bacteria. “To do that, we have to identify the presence of resistant germs in hospitals as quickly as possible. One way to do that is by systematically screening all patients who’ve been abroad and then isolating carriers,” says Thierry Calandra.
That is what has driven Patrice Nordmann and his colleague Laurent Poirel to develop a diagnostic test that can detect resistant strains of the Acinetobacter baumannii bacterium in less than two hours, as opposed to the previous two-day wait. A Geneva-based team has de- signed a tool used to detect tuberculosis strains resistant to the antibiotic rifampicin in two hours instead of two to eight weeks.
But to really finish off these super-bacteria, we need new antibiotics. And unfortunately, the pharmaceutical industry seems to have other priorities in mind.
“Drug manufacturers haven’t developed any new products for years, because it doesn’t bring in enough money,” says Didier Pittet.
“It’s much more profitable to develop drugs for Alzheimer’s or diabetes, which patients will have to take for the rest of their lives”.
Didier Pittet believes we’ll eventually need to create new funding models.
“Antibiotics could be listed as UNESCO Intangible Cultural Heritage, which would draw in subsidies from the World Bank or European Union”, he says.
In 2015, the Swiss Confederation announced its new National Research Programme on Antimicrobial Resistance backed with total funding of 20 million Swiss francs. “This programme aims to develop new ways of treating resistant bacteria”, says Karin Wäfler. The FOPH said that it would provide further details between now and mid- 2016 while keeping the public regularly informed of its progress. The fight against super-bacteria has begun. Now all we have to do is win it. ⁄
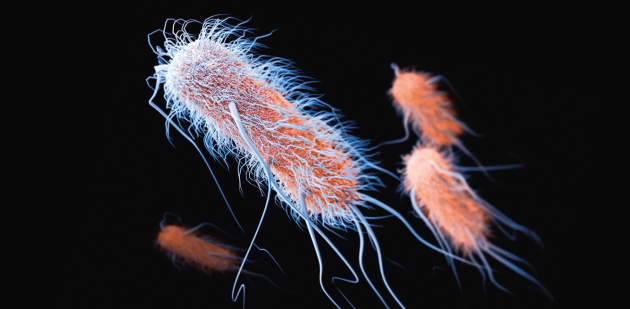
Extended-spectrum beta-lactamases, here covered in pili (hair) and flagella, are enzymes that can develop resistance to certain antibiotics.
Extended-spectrum beta-lactamases are enzymes produced by certain gut bacteria such as E. coli and K. pneumoniae. Discovered for the first time in France and Germany in the mid-1980s, ESBL provide resistance to beta-lactam antibiotics, which are the most commonly used. These enzymes appeared in Switzerland in the early 2000s. In 2010, 4.8% of patients admitted to the Geneva University Hospitals and 5.8% of those coming to the Zurich Hospital were carriers.
Staphylococcus aureus is a bacterium found in the skin of 30% of the human population. It can cause infections in the bloodstream, soft tissue and joints. The bug has developed resistance to the first-line anti- biotic meticillin, making it one of the most common causes of hospital-acquired infections. Its incidence has begun to subside over the past ten years, however, with the prevalence of resistant strains dropping from 12.7% in 2004 to 5% in 2013.
Carbapenemases is another enzyme produced by intestinal microflora that deactivates carbapenems, the latest generation of antibiotics. Patients infected with this superbug are left
with only one option, colistin. Discovered in 1949, this drug is used as a last-resort antibiotic due to its toxicity. For the time being, all cases of carbapenemases reported in Switzerland have been imported from either Mediterranean countries or the Indian sub-continent. But the number of strains present in Switzerland has exploded from less than 15 in 2009 to more than 400 to day.