Major discoveries have catapulted the gut to the centre stage of science. Unlocking the secrets of its bacteria could revolutionise the treatment of certain diseases.
What are the intestines? “A vast population of bacteria, which is the sum of the decisions we make, the food we ingest, the environment we live in... It’s like our own personal collection of Pokemons!” That’s how Giulia Enders describes this organ, with confidence and humour, in a “science slam” show that she has presented on several stages in her native tongue, German. The young medical student then published the book Darm mit Charme (Gut: The Inside Story of Our Body’s Most Underrated Organ) that has become one of the biggest surprise hits of the year in German bookshops (read the interview p. 26).
This success reflects the growing interest in the gut from both the general public and the medical community. Long considered a simple digestive organ, this five-metre long tube between the stomach and the anus actually plays a key role in the organism.
A major advance has captured everyone’s attention. Molecular genetics. “The progress made in this field now enables us to describe the genome of the 100 trillion bacteria that colonise our digestive tract,” says Jacques Schrenzel, head of the bacteriology laboratory at Geneva University Hospitals (HUG). “Now we need to understand exactly ‘who does what’ in this bacterial community called the ‘gut microbiota.’”
A number of studies have confirmed a hypothesis that the scientific community now acknowledges as fact: “Having a diverse and balanced microbiota prevents and protects against a number of disorders,” says Michel Maillard, a gastroenterologist at Lausanne University Hospital (CHUV). “Finding ways to restore diversity in the bacteria, fungi and other microorganisms in the intestines is now the crusade led by hundreds of researchers across the world.”
Because these little beings do not sit around idle. They actively contribute to the balance of physiological functions, and therefore the overall health, of their host. “New information and treatments will emerge as research advances on the microbiota,” says Jacques Schrenzel. “These discoveries will have such a huge impact on the care provided for many conditions that all the current medical books will have to be updated.” And this is not restricted to intestinal disorders. Obesity, alcohol addiction, depression and bulimia are just some of the diseases that could benefit from these advances in the future.
What do these 2 kg of microbes do in the gut? They are obviously
involved in the digestive process. “Microbes play a role in converting food into nutrients and energy and in synthesizing essential vitamins,” says Francisca Joly Gomez, gastroenterologist at Beaujon Hospital in Clichy, France.
But their action goes well beyond that. They seem to be in constant dialogue with other components of the organism, especially the brain. “We’ve known for a long time that the brain sends information to the intestines, but now we also know that it’s a bidirectional relationship. Information is exchanged in both directions,” says the specialist who published a book last year called The Gut, Our Second Brain (original title: L’intestin, notre deuxième cerveau).
The enteric nervous system is responsible for sending and receiving information. “More than 100 million neurons are concentrated and connected to one another in the gastrointestinal wall.” So what can the intestines actually “say” to the brain? “For example, it can send the brain ‘pain’ messages. Gas is generated in the intestines by the fermentation of food. Intestinal bloating can cause visible pain signals in the brain,” the specialist explains.
The connection between the brain and the gut has led to major discoveries about Parkinson’s disease. Researchers from the French Institute of Health and Medical Research (Inserm) have shown that the abnormalities in the brain neurons of patients with this degenerative condition have “equivalent” abnormalities in the neurons of the digestive tract. In the future, the disease could potentially be identified and diagnosed early through an intestinal biopsy. “The defective neurons in the intestines can apparently be detected before their counterparts in the brain.”
Over the past ten years, mounting evidence has confirmed the link between Parkinson’s disease and the patient’s gut. The latest research includes a study from the University of Helsinki, which found that patients with Parkinson’s disease have much less bacteria from the Prevotellaceae family in their gut. “In addition, we will have to see if these changes in the bacterial ecosystem are apparent before the onset of motor symptoms,” says neurologist Filip Scheperjans. “We may finally perhaps treat Parkinson’s by focusing on gut microbiota.”
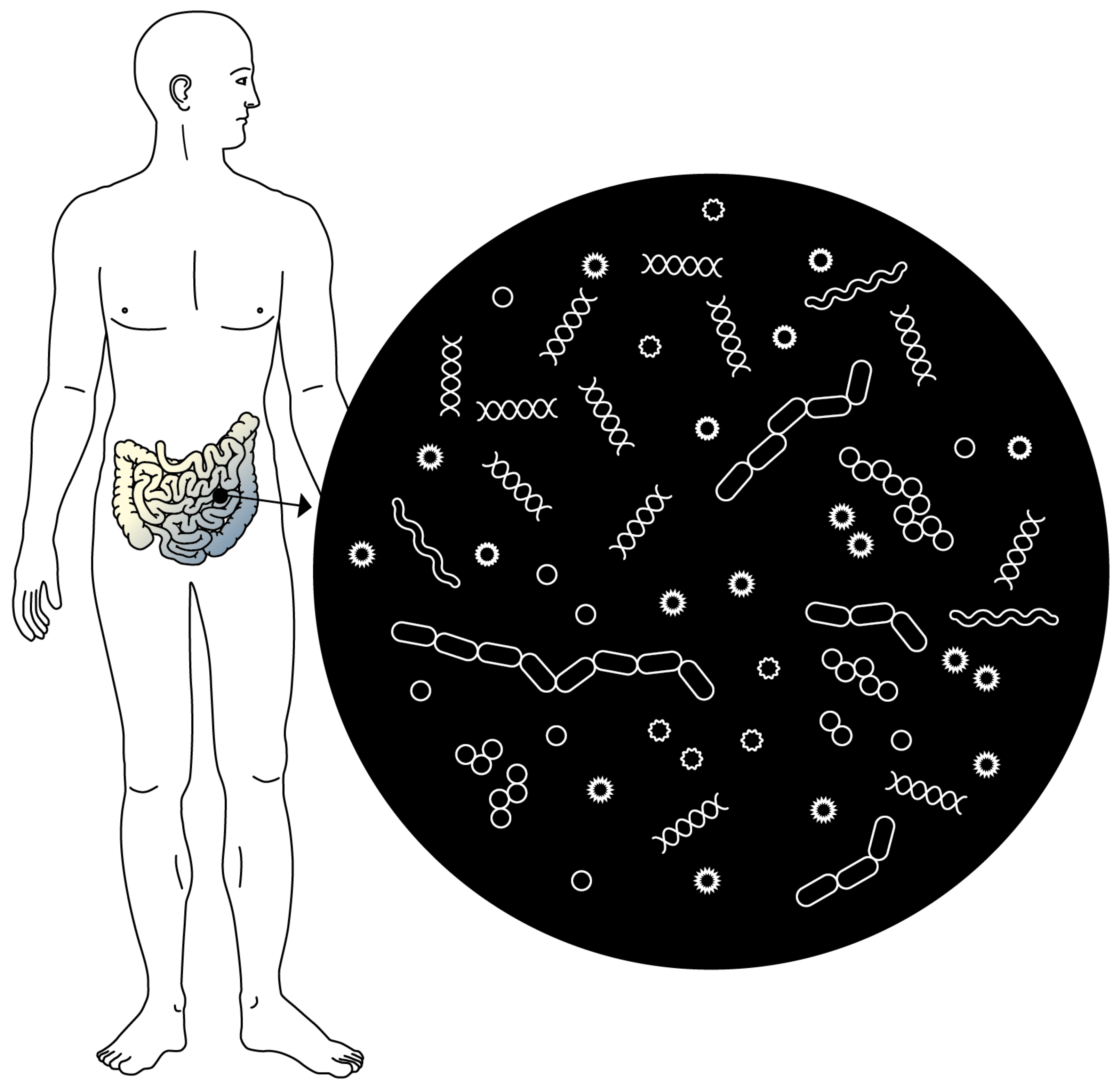
An organ in its own right. The microbiota is the ecosystem made up of billions of microorganisms that colonise the intestines. Today it is considered an organ in its own right.
“Protective barrier”
The intestines communicate not only with the brain, but also the immune system. “The bacteria of the microbiota send signals to the receptors located on the cells of the gastrointestinal wall, which in turn sends a signal to our immune cells to help them exclude pathogens that might try to colonise the intestines,” says Francisca Joly Gomez.
Exploring the microbiota’s role of “protective barrier” opens up new possibilities for treatments of certain conditions, such as food allergies. “Tests on mice that had been administered peanut allergens showed that the presence of the bacteria Clostridium in their gut microbiota blocked the allergic reaction,” says Cathryn Nagler, from the University of Chicago, who led the study. “This leads us to believe that these specific bacteria may act through certain immune cells and prevent the proteins responsible for allergic reactions from entering the bloodstream.”
The Spanish National Research Council published its research findings in October 2014 in the journal of the American Society for Microbiology, highlighting further links between the microbiota and certain autoimmune diseases. The gut microbiota of patients with lupus, a chronic disease that attacks healthy tissue, shows an imbalance in the ratio between two dominant types of microorganisms in the human gut, revealing more Bacteroides than Firmicutes.
Efforts are growing worldwide to make the most of these discoveries (MetaHIT, MetaGenoPolis and MyNewGut projects in Europe, Human Microbiome Project in the United States), and findings are regularly published in the most prestigious scientific journals.
“Small intestinal bacterial overgrowth has finally been demonstrated and recognised,” says Alain Schoepfer, gastroenterologist at the CHUV. “This refers to abnormally high numbers of bacteria in the organ that can cause severe abdominal pain, bloating or diarrhoea. We now know that administering an antibiotic used to treat urinary infections can effectively improve the patient’s health.”
Several studies have also linked gut microbiota to metabolic diseases such as obesity and diabetes. An international team headed by Jeffrey Gordon from the Washington University School of Medicine in the United States showed that introducing the gut microbiota from an obese individual into mice, the animals grew fatter as well. Conversely, the microbiota of a thin person keeps them lean. The researcher explained in the journal Nature that gut bacteria have an impact on regulating fat storage in adipose tissue.
The permanent dialogue between the gut and the brain has made scientists wonder if the microbiota also influences the behaviour of its host. To answer that question, researchers from University College Cork in Ireland tested “axenic” mice, i.e. bacteria-free, raised in a sterile environment since birth. The mice showed behavioural changes. They preferred to stay in an empty cage instead of with other mice. Their attitude changed once bacteria were injected into their digestive system, and they gladly shared their living space.
“All of this work on mice is valuable and provides us with interesting ideas for further research,” says Michel Maillard. “We shouldn’t get too excited just yet. Humans will not necessarily react the same way.” Alcohol dependence and bulimia are other diseases suspected to be linked to the microbiota. Research led by a team from the Université catholique de Louvain reported that alcoholic patients with an altered gut microbiota showed more depression, anxiety and alcohol craving than alcoholics with a “normal” microbiota.
The protein ClpB could cause eating disorders. “This protein is produced by certain bacteria, such as Escherichia Coli, which are naturally present in the intestinal flora,” says Serguei Fetissov, from the French Institute of Health and Medical Research (Inserm) of the University of Rouen. “The protein is secreted when the bacteria are subjected to stress. ClpB has anorexigenic properties, meaning that it reduces the appetite and triggers an immune reaction in which antibodies are produced against it. These will also bind to the satiety hormone because of its structural homology and result in anorexia or bulimia.”
To know more
30 partnerships in
15 different countries came together for the My New Gut project. Its objective is to understand the role played by the microbiota in human metabolism and developing food that is good for gut microorganisms.
The Human Microbiome Project (HMP), launched by the US National Institutes of Health, aims to collect microorganisms in the human body and analyse their genome to understand how they affect human health.
MetaGenoPolis (MGP) is a French project set up to understand how the human gut microbiota impacts health and disease.
“The long-term objective is personalised prevention or treatment of certain diseases based on patients’ specific combination of bacterial strains,” says the Geneva-based microbiologist Jacques Schrenzel.
Several treatments developed from microbiota research have been applied to certain patients. The most widespread is the faecal transplant, i.e. placing fresh stool from a healthy donor by colonoscopy.“We began performing this procedure in 2014 on patients suffering from recurring Clostridium difficile colitis,” says Michel Maillard. “These bacteria are sometimes resistant to antibiotics and need to be eliminated because they cause severe diarrhoea, which can be serious for the patients.”
“The response rate to this treatment is close to 90%,” says Alain Schoepfer from the CHUV. “In less than two weeks, patients are doing better.” The stool donor is generally someone close to the patient. “Before the transplant, donors undergo microbiology screening. They must be healthy and not carry Clostridium difficile.”
In Geneva, the HUG will also perform this procedure starting in the spring of 2015. “We will first use this method to treat patients with an infection resistant to Clostridium difficile, but we hope that we can also apply it to patients suffering from inflammatory bowel diseases such as Crohn’s disease or ulcerative colitis,” says Jacques Schrenzel.
Although there is no recommendation against having faecal transplants, nothing is known about the long-term side effects. “We inject a ‘black box’ of several billion microbes into the patient,” Alain Schoepfer says. “We know that it will treat a targeted condition, but will this ‘new’ microbiota cause another illness? We explain these risks to patients, who must give us their consent.”
Most importantly, this treatment is, in its entirety, much less invasive than an organ transplant. “Small bowel transplantation is very rare,” says Nicolas Desmartines, chief of the Service of Visceral Surgery at the CHUV. The gut is a very sensitive organ, easily rejected and infected. If we operate on the small intestine, it starts forming ‘adhesions’, a type of obstruction that prevents digestion and causes severe abdominal pain. The organ can stop working for two or three days. Colon transplants are rare because the organ contains such an extraordinary number of bacteria that is not suited to transplants. Plus, the small intestine can take over some of its functions.”
Food is always another option for taking in “good” bacteria. “Probiotics are live microorganisms that are already added to some products such as yoghurt,” says Michel Maillard. “Today, food industry giants are trying to introduce more bacteria and yeasts into food. In the future, we may find them in our supermarkets.”
“If one day we determine the ‘ideal’ microbiota, we could develop a treatment in the form of a pill to restore microbiota diversity and above all microbial balance,” says the gastroenterologist Francisca Joly Gomez. “That’s still a long way away. We have already made a huge leap forward by accepting a change in paradigm: we don’t always have to destroy the ‘bad’ bacteria with a blast of antibiotics. Instead, we need to learn to live in harmony with them.”
Intestine or intestines?
The plural is more appropriate because there are actually two organs: the small intestine, a tube about six metres long that absorbs nutrients, and the large intestine
or colon. The colon measures between 80 cm and 150 cm in
length, and its main functions are
to store waste, absorb certain vitamins and water to form stool. Bacteria colonise the entire digestive tract, but the vast majority
of the microbiota are found
in the colon.
The surface area of the intestines, the equivalent
of two tennis courts
The percentage of
the microbiota present
in the colon. The rest
is spread throughout
the digestive tract.
The number of bacterial species identified in the human gut microbiota. There are only 150 to
170 predominant species per individual.
The number of genes
in the gut microbiota, 150 times more than
the number of genes
in the human genome.
The number of bacteria that colonise the gut, ten times more than the number of human cells.
The total weight
of all gut bacteria.
true One-third of our gut microbiota is shared by most individuals, but the other two-thirds are specific to each of us.
false The gut microbiota starts developing at birth. A newborn’s digestive tract goes from being sterile inside the uterus to being rapidly colonised through contact with the microorganisms of the mother (vaginal, faecal, skin, etc.) and the environment where the birth takes place. After that, the composition of the gut microbiota depends on how the infant is fed. Scientists believe that the microbiota stabilises around the age of three and continues to develop steadily over the person’s lifetime.
true/false Many studies
have shown the benefits of probiotics on the gut flora. However, research led by French microbiologist Didier Raoult showed the unwanted effects
of these bacteria. They can
lead to obesity.
Several studies, such as the one by the National Institute for Agricultural Research (Institut national de la recherche agronomique), have shown that having “poor” gut bacteria is associated with being overweight. This lack of diversity increases the risk of developing complications due to obesity, such as diabetes or cardiovascular diseases.
Research led by the Université catholique de Louvain reported that some alcoholics present alterations in the composition of their gut microbiota. This discovery opens up new possibilities for therapies focusing on the intestines and not just the brain in treating alcohol dependence.
Researchers from
the California Institute of Technology improved symptoms of autism in mice by administering a human gut bacterium, “Bacteroides fragilis”, known for contributing to tightness in the colon wall.
Patients with chronic inflammatory bowel diseases have excessive concentrations of potentially pathogenic bacteria and a lower proportion and number of species of a beneficial category of bacteria called “Firmicutes”.